2022
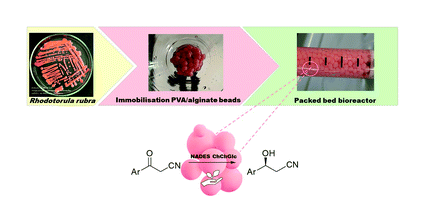
Continuous-flow stereoselective reduction of prochiral ketones in a whole cell bioreactor with natural deep eutectic solvents
Annunziata, F.| Guaglio, A.| Conti, P.| Tamborini, L.| Gandolfi, R.
Green Chem., 24, 950 (2022)
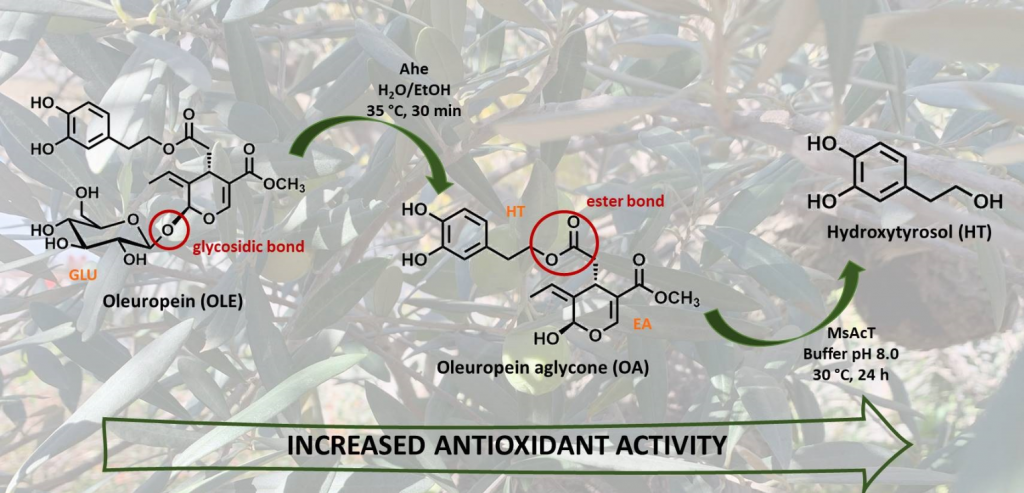
Efficient 2-Step Enzymatic Cascade for the Bioconversion of Oleuropein into Hydroxytyrosol
Catinella, G.| Donzella, S.| Borgonovo, G.| Dallavalle, S.| Contente, M.L.| Pinto, A
Antioxidants, 11(2), 260 (2022)
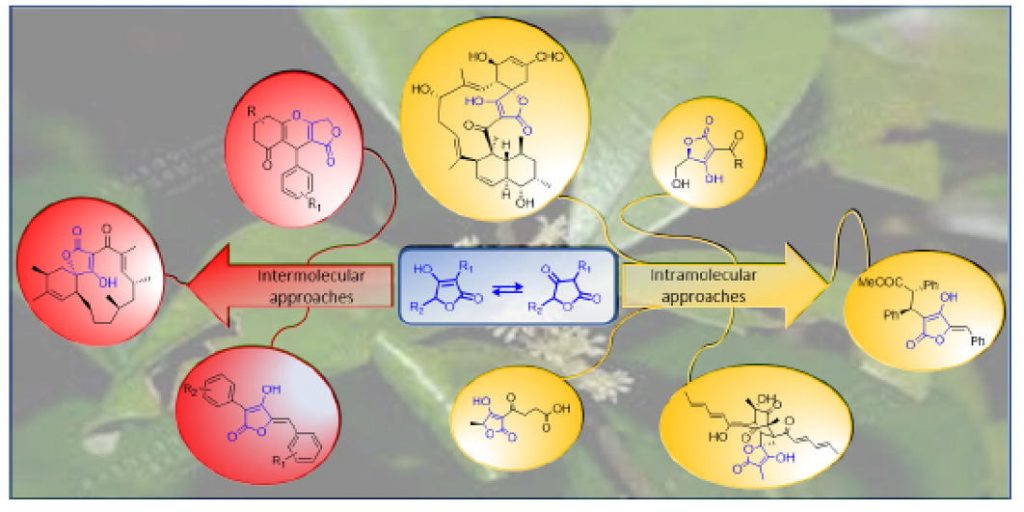
Recent advances in the synthesis of naturally occurring tetronic acids
Princiotto, S.| Jayasinghe, L.| Dallavalle, S.
Bioorganic Chemistr,y 119, 105552 (2022)
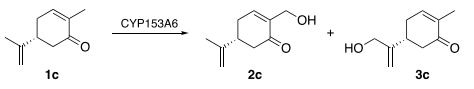
Whole cells of recombinant CYP153A6-E. coli as biocatalyst for regioselective hydroxylation of monoterpenes
Cannazza, P.| Rabuffetti, M.| Donzella, S.| De Vitis, V.| Contente, M.L.| de Oliveira, M.C.F.| de Mattos, M.C.| Barbosa, F.G.| de Souza Oliveira, R.P.| Pinto, A.| Molinari, F.| Romano, D.
AMB Express, 12:48 (2022)
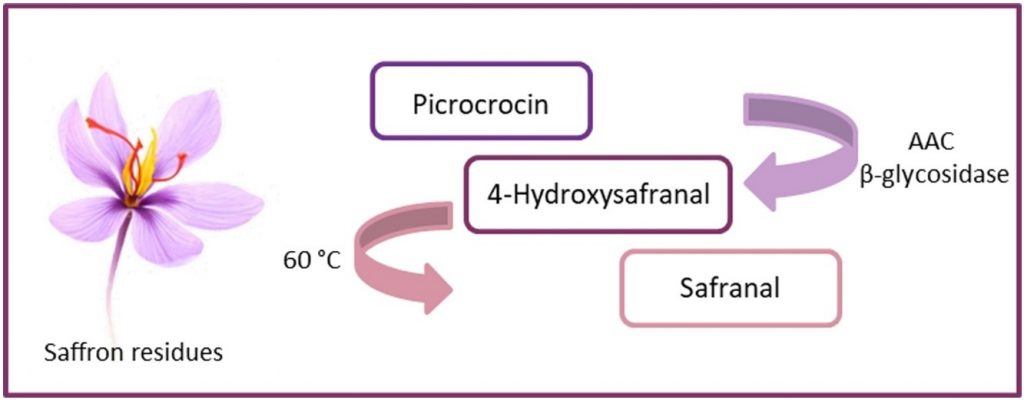
From saffron residues to natural safranal: Valorization of waste through a β-glucosidase
Catinella, G.| Borgonovo, G.| Dallavalle, S.| Contente, M.L.| Pinto, A.
Food and Bioproducts Processing, 131, 144–148 (2022)
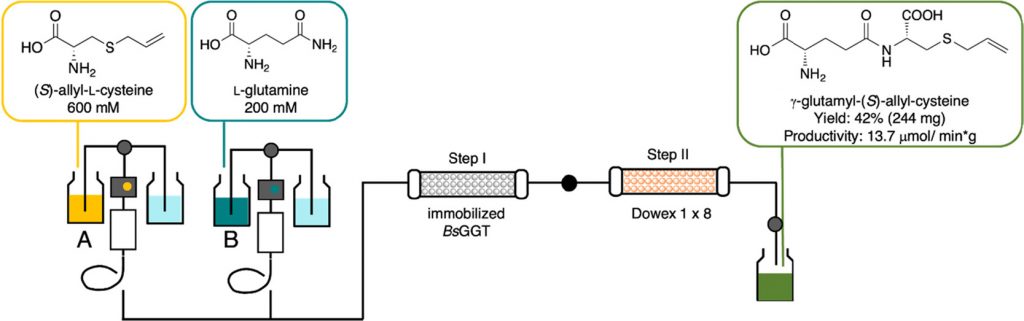
From Batch to Continuous Flow Bioprocessing: Use of an Immobilized γ-Glutamyl Transferase from B. subtilis for the Synthesis of Biologically Active Peptide Derivatives
Robescu, M.S.| Annunziata, F.| Somma, V.| Calvio, C.| Morelli, C.F.| Speranza, G.| Tamborini, L.| Ubiali, D.| Pinto, A.| Bavaro, T.
J. Agric. Food Chem., 70, 13692−13699 (2022)
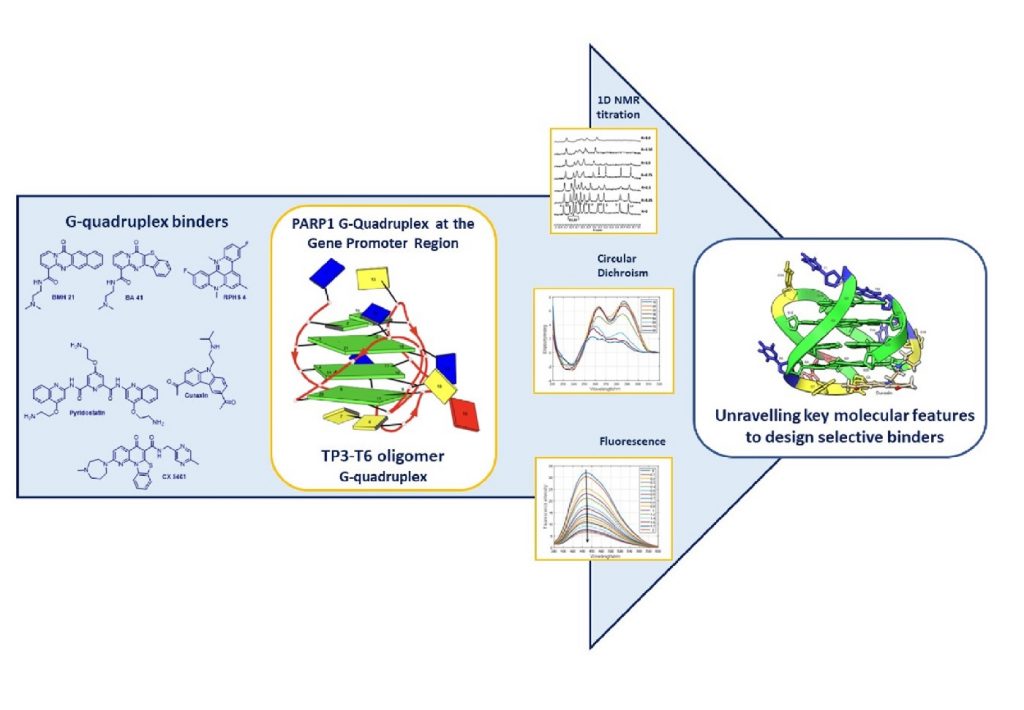
Exploring the Interaction of G-quadruplex Binders with a (3 + 1) Hybrid G-quadruplex Forming Sequence within the PARP1 Gene Promoter Region
Mazzini, S.| Princiotto, S.| Artali, R.| Musso, L.| Aviñó, A.| Eritja, R.| Gargallo, R.| Dallavalle, S.
Molecules , 27(15), 4792 (2022)
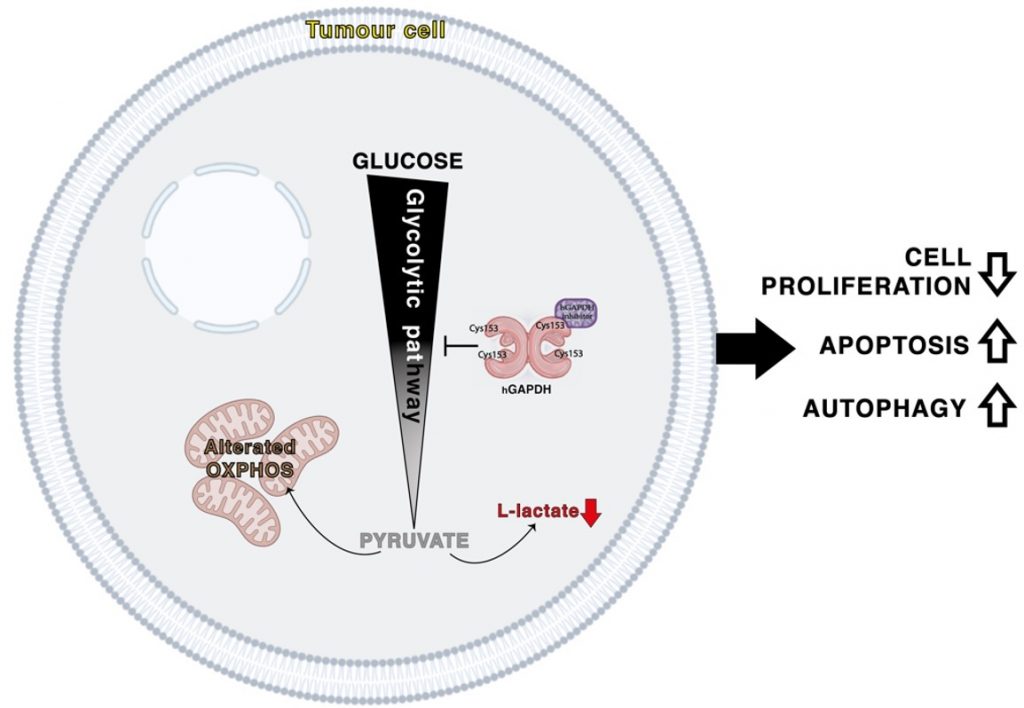
3-Bromo-Isoxazoline Derivatives Inhibit GAPDH Enzyme in PDAC Cells Triggering Autophagy and Apoptotic Cell Death
Pacchiana, R.| Mullappilly, N.| Pinto, A.| Bova, S.| Forciniti, S.| Cullia, G.| Pozza, E.D.| Bottani, E.| Decimo, I.| Dando, I.| Bruno, S.| Conti, P.| Donadelli, M.
Cancers, 14(13), 3153 (2022)
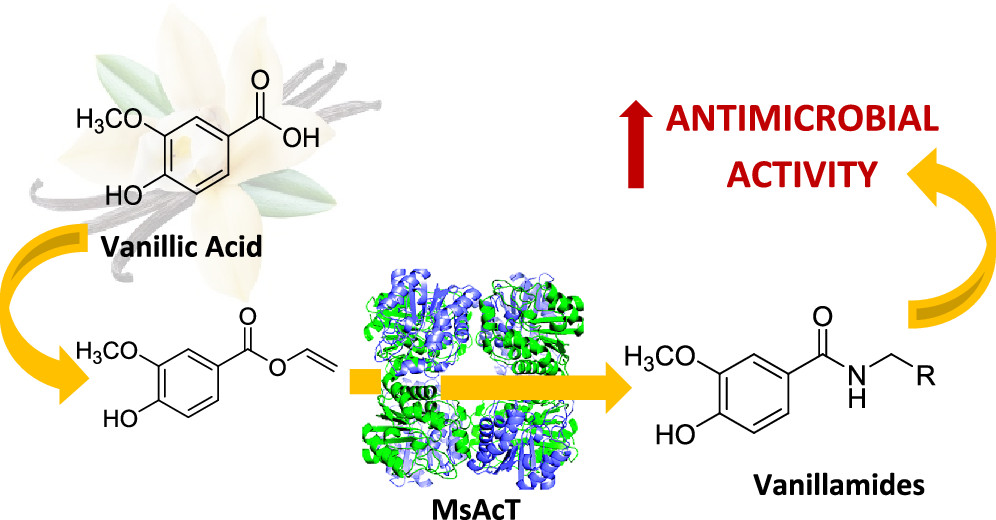
Biocatalyzed Synthesis of Vanillamides and Evaluation of Their Antimicrobial Activity
Pinna, C.| Martino, P.A.| Meroni, G.| Sora, V.M.| Tamborini, L.| Dallavalle, S.| Contente, M.L.| Pinto, A.
J. Agric. Food Chem., 70, 223−228 (2022)

Enzymatic continuous-flow preparation of nature-inspired phenolic esters as antiradical and antimicrobial agents
Annunziata, F.| Contente, M.L.| Anzi, V.| Donzella, S.| Conti, P.| Molinari, F.| Martino, P.A.| Meroni, G.| Sora, V.M.| Tamborini, L.| Pinto, A.
Food Chemistry, 390, 133195 (2022)

Enzymatic amide bond formation: synthesis of aminooxo-acids through a Mycobacterium smegmatis acyltransferase
Christodoulou, M.S.| Contente, M.L.| Dallavalle, S.| Pinto, A.
Green Chem., 24, 4432-4436 (2022)
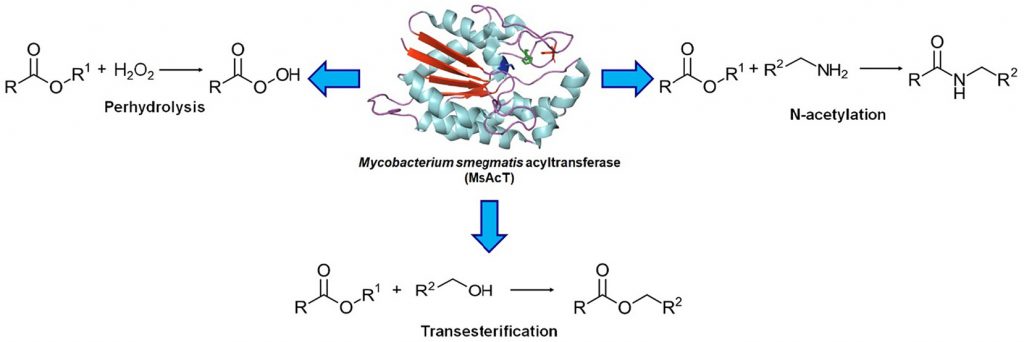
Mycobacterium smegmatis acyltransferase: The big new player in biocatalysis
Cannazza, P.| Donzella, S.| Pellis, A.| Contente, M.L.
Biotechnology Advances, 59, 107985 (2022)

Mimicking Natural Metabolisms: Cell-Free Flow Preparation of Dopamine
Donzella, S.| Colacicco, A.| Nespoli, L.| Contente, M.L.
ChemBioChem, 23, e202200462 (2022)

Investigation of the Interaction between Aloe vera Anthraquinone Metabolites and c-Myc and C-Kit G-Quadruplex DNA Structures
Dallavalle, S.| Artali, R.| Princiotto, S.| Musso, L.| Borgonovo, G.| Mazzini, S.
Int. J. Mol. Sci., 23(24), 16018 (2022)

Screening methods for enzyme-mediated alcohol oxidation
Contente, M.L.| Marzuoli, I.| Iding, H.| Wetzl, D.| Puentener, K.| Hanlon, S.P.| Paradisi, F.
Scientific Reports, 12, 3019 (2022)
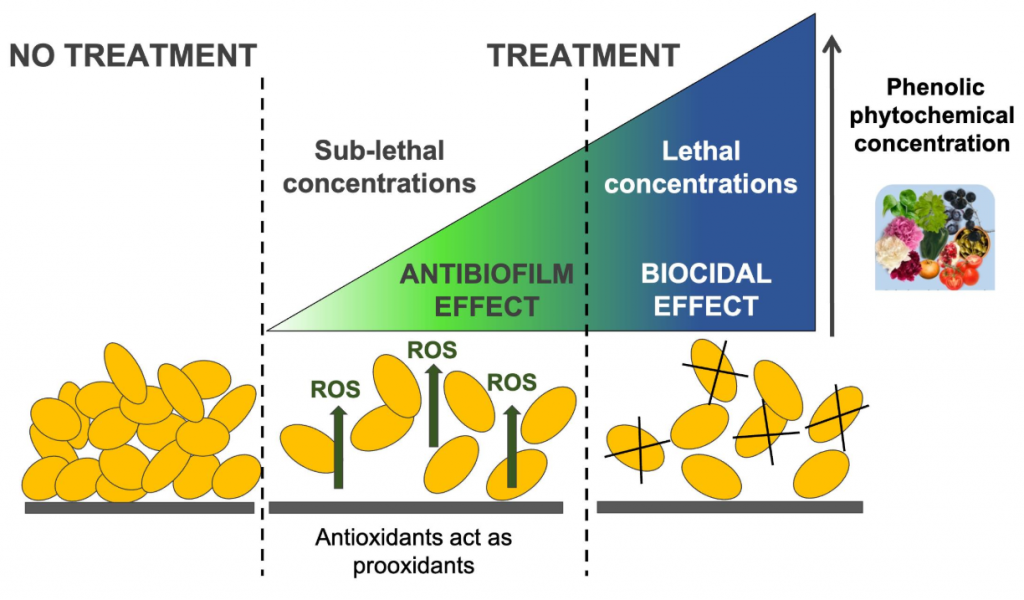
Correlation between Perturbation of Redox Homeostasis and Antibiofilm Capacity of Phytochemicals at Non-Lethal Concentrations
Christodoulou, M.S.| Villa, F.| Pinto, A.| Cappitelli, F.
Antioxidants, 11(12), 2451 (2022)
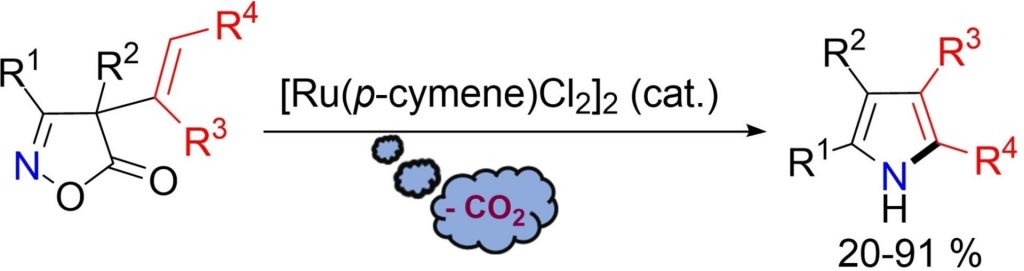
Ruthenium-Catalyzed Decarboxylative Rearrangement of 4-Alkenyl-isoxazol-5-ones to Pyrrole Derivatives
Molteni, L.| Loro, C.| Christodoulou, M.S.| Papis, M.| Foschi, F.| Beccalli, E.M.| Broggini, G.
Eur. J. Org. Chem., e20220049 (2022)

Antitumor activity of novel POLA1-HDAC11 dual inhibitors
Dallavalle, S.| Musso, L.| Cincinelli, R.| Darwiche, N.| Gervasoni, S.| Vistoli, G.| Guglielmi, M.B.| La Porta, I.| Pizzulo, M.| Modica, E.| Prosperi, F.| Signorino, G.| Colelli, F.| Cardile, F.| Fucci, A.| D’Andrea, E.L.| Riccio, A.| Pisano, C.
Eur. J. Med. Chem., 228, 113971 (2022)
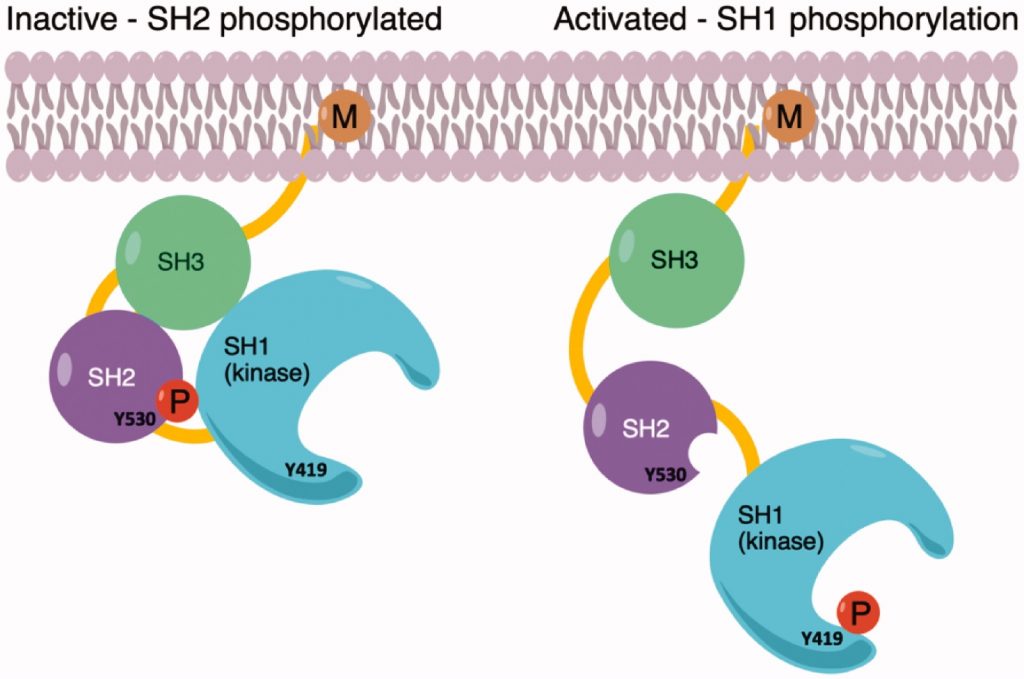
Synthesis and biological activity evaluation of 3-(hetero) arylideneindolin-2-ones as potential c-Src inhibitors
Princiotto, S.| Musso, L.| Manetti, F.| Marcellini, V.| Maga, G.| Crespan, E.| Perini, C.| Zaffaroni, N.| Beretta, G.L.| Dallavalle, S.
J. Enzyme Inhib. Med. Chem., 37(1), 2382–2394 (2022)

Flow Synthesis of Nature-Inspired Mitochondria-Targeted Phenolic Derivatives as Potential Neuroprotective Agents
Pecora, D.| Annunziata, F.| Pegurri, S.| Picone, P.| Pinto, A.| Nuzzo, D.| Tamborini, L.
Antioxidants, 11(11), 2160 (2022)
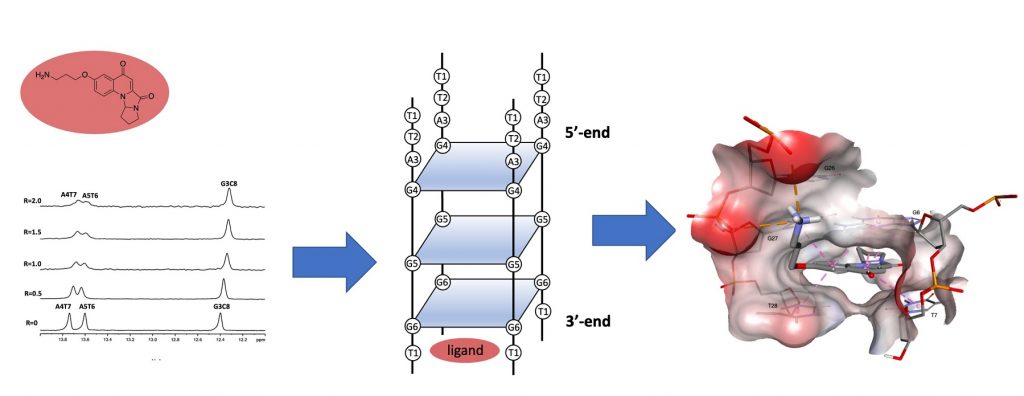
Synthesis and Investigation of the G-Quadruplex Binding Properties of Kynurenic Acid Derivatives with a Dihydroimidazoquinoline-3,5-dione Core
Mazzini, S.| Princiotto, S.| Musso, L.| Passarella, D.| Beretta, G.L.| Perego, P.| Dallavalle, S.
Molecules 27(9), 2791 (2022)

Fatty Acids/Tetraphenylethylene Conjugates: Hybrid AIEgens for the Preparation of Peptide-Based Supramolecular Gels
Impresari, E.| Bossi, A.| Lumina, E.M.| Ortenzi, M.A.| Kothuis, J.M.| Cappelletti, G.| Maggioni, D.| Christodoulou, M.S.| Bucci, R.| Pellegrino, S.
Front. Chem. 10, 927563 (2022)
