2020
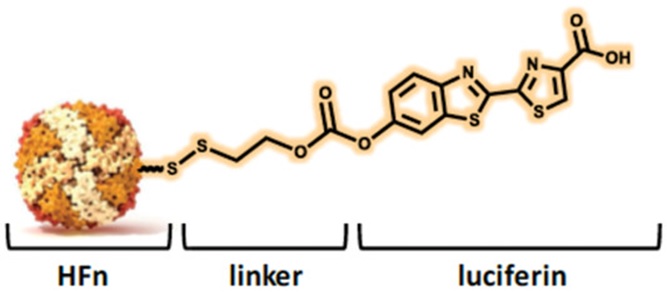
Engineered Ferritin Nanoparticles for the Bioluminescence Tracking of Nanodrug Delivery in Cancer
Bellini, M.| Riva, B.| Tinelli, V.| Rizzuto, M.A.| Salvioni, L.| Colombo, M.| Mingozzi, F.| Visioli, A.| Marongiu, L.| Frascotti, G.| Christodoulou, M.S.| Passarella, D.| Prosperi, D.| Fiandra, L.
Small, 16, 2001450, (2000)
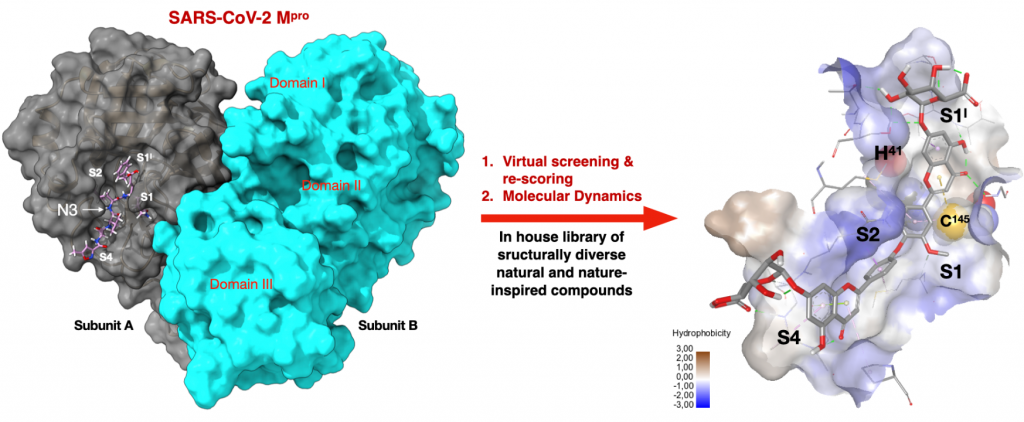
Putative SARS-CoV-2 Mpro Inhibitors from an In-House Library of Natural and Nature-Inspired Products: A Virtual Screening and Molecular Docking Study
Mazzini, S.| Musso, L.| Dallavalle, S.| Artali, R.
Molecules, 25 (16), 3745 (2020)

Urinary TMAO levels are associated with the taxonomic composition of the gut microbiota and with the choline TMA-lyase gene (cutC) harbored by enterobacteriaceae
Dalla Via, A.| Gargari, G.| Taverniti, V.| Rondini, G.| Velardi, I.| Gambaro, V.| Visconti, G.L.| De Vitis, V.| Gardana, C.| Ragg, E.| Pinto, A.| Riso, P.| Guglielmetti, S.
Nutrients, 12(1), 62, (2000)
DOI: 10.3390/nu12010062

A small library of chalcones induce liver cancer cell death through Akt phosphorylation inhibition
Sahin, I.D.| Christodoulou, M.S.| Guzelcan, E.A.| Koyas, A.| Karaca, C.| Passarella, D.| Cetin-Atalay, R.
Sci. Rep. 10, 11814 (2020)

Continuous preparation of flavour-active acetate esters by direct biocatalytic esterification
Chiarelli Perdomo, I.| Letizia Contente, M.| Pinto, A.| Romano, D.| Fernandes, P.| Molinari, F.
Flavour Fragr J., 35,190–196 (2020)
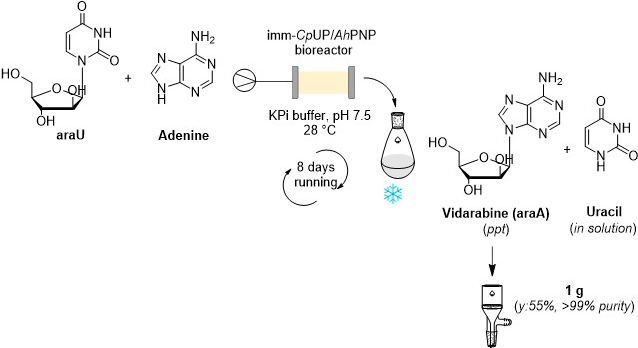
An enzymatic flow-based preparative route to vidarabine
Tamborini, L.| Previtali, C.| Annunziata, F.| Bavaro, T.| Terreni, M.| Calleri, E.| Rinaldi, F.| Pinto, A.| Speranza, G.| Ubiali, D.| Conti, P.
Molecules , 25(5), 1223 (2020)
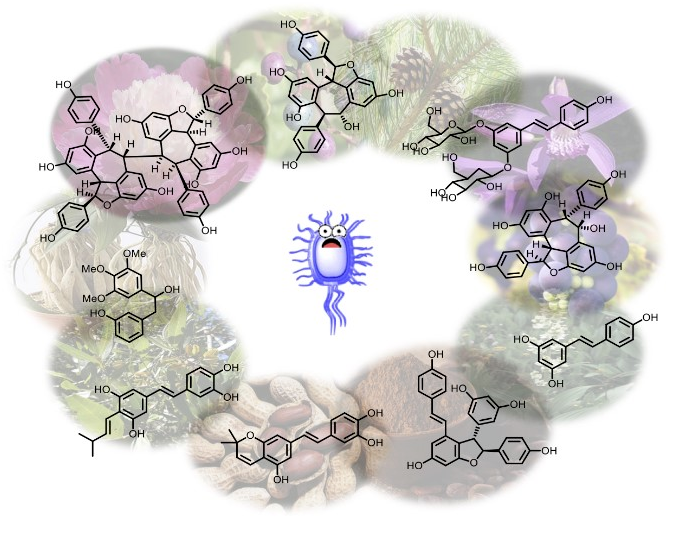
Stilbenoids: A natural arsenal against bacterial pathogens
Mattio, L.M.| Catinella, G.| Dallavalle, S.| Pinto, A.
Antibiotics, 9(6), 336 (2020)
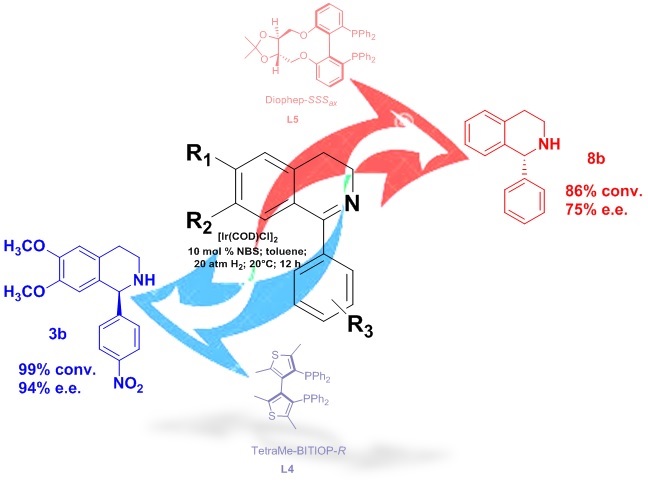
Asymmetric hydrogenation of 1-aryl substituted-3,4-dihydroisoquinolines with iridium catalysts bearing different phosphorus-based ligands
Facchetti, G.| Christodoulou, M.S.| Binda, E.| Fusè, M.| Rimoldi, I.
Catalysts, 10(8), 914, (2020)
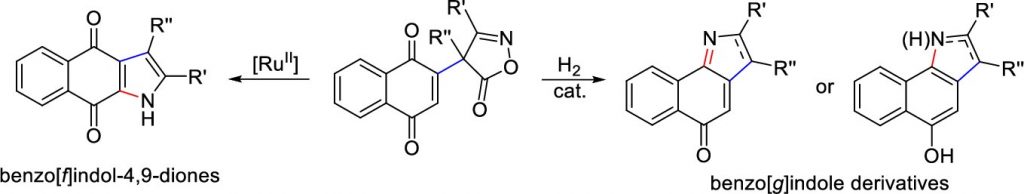
Divergent Conversion of 4-Naphthoquinone-substituted 4 H-Isoxazolones to Different Benzo-fused Indole Derivatives
Christodoulou, M.S.| Giofrè, S.| Beccalli, E.M.| Foschi, F.| Broggini, G.
Org. Lett. 2020, 22, 7, 2735–2739
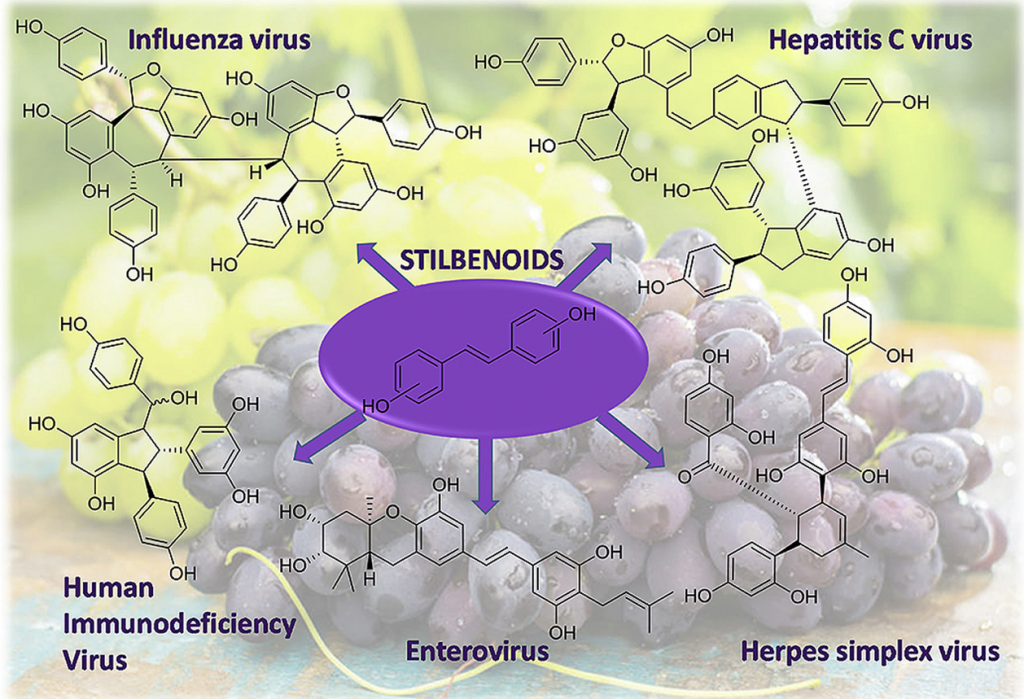
Natural and nature-inspired stilbenoids as antiviral agents
Mattio, L.M.| Catinella, G.| Pinto, A.| Dallavalle, S.
Eur. J. Med. Chem., 202 ,112541 (2020)

Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors
Dallavalle, S.| Dobričić, V.| Lazzarato, L.| Gazzano, E.| Machuqueiro, M.| Pajeva, I.| Tsakovska, I.| Zidar, N.| Fruttero, R.
Drug Resist Updat. 50:100682 (2020)

Embelin as lead compound for new neuroserpin polymerization inhibitors
Visentin, C.| Musso, L.| Broggini, L.| Bonato, F.| Russo, R.| Moriconi, C.| Bolognesi, M.| Miranda, E.| Dallavalle, S.| Passarella, D.| Ricagno, S.
Life, 10(7), 111 (2020)
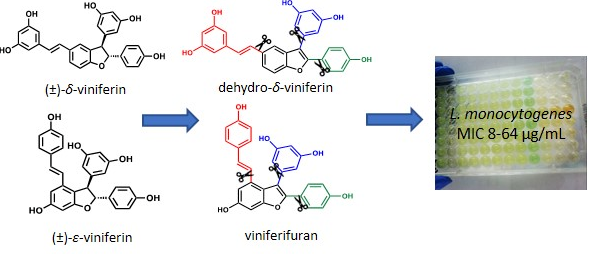
Structural requirements of benzofuran derivatives dehydro-δ-and dehydro-ε-viniferin for antimicrobial activity against the foodborne pathogen listeria monocytogenes
Catinella, G.| Mattio, L.M.| Musso, L.| Arioli, S.| Mora, D.| Beretta, G.L.| Zaffaroni, N.| Pinto, A.| Dallavalle, S.
Int. J. Mol. Sci., 21(6), 2168 (2020)
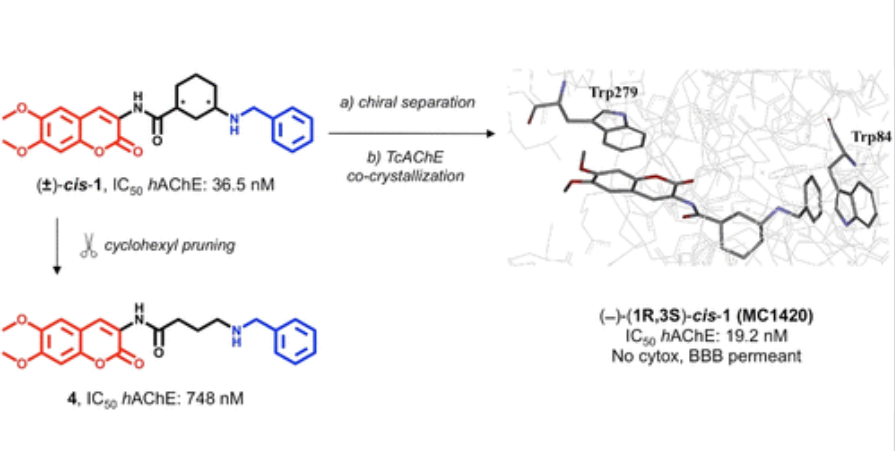
Chiral separation, X-ray structure, and biological evaluation of a potent and reversible dual binding site ache inhibitor
Catto, M.| Pisani, L.| Mora, E.D.L.| Belviso, B.D.| Mangiatordi, G.F.| Pinto, A.| De Palma, A.| Denora, N.| Caliandro, R.| Colletier, J.-P.| Silman, I.| Nicolotti, O.| Altomare, C.D.
ACS Med. Chem. Lett., 11, 5, 869-876

Uncommon overoxidative catalytic activity in a new halo-tolerant alcohol dehydrogenase
Contente, M.L.| Fiore, N.| Cannazza, P.| Roura Padrosa, D.| Molinari, F.| Gourlay, L.| Paradisi, F.
ChemCatChem, 12, 5679 – 5685 (2020)
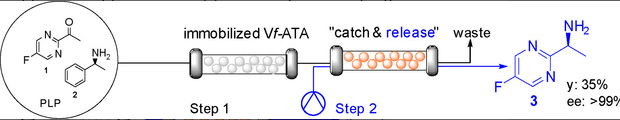
Use of Immobilized Amine Transaminase from Vibrio fluvialis under Flow Conditions for the Synthesis of (S)-1-(5-Fluoropyrimidin-2-yl)-ethanamine
Semproli, R.| Vaccaro, G.| Ferrandi, E.E.| Vanoni, M.| Bavaro, T.| Marrubini, G.| Annunziata, F.| Conti, P.| Speranza, G.| Monti, D.| Tamborini, L.| Ubiali, D.
ChemCatChem, 12, 1359 – 1367 (2020)
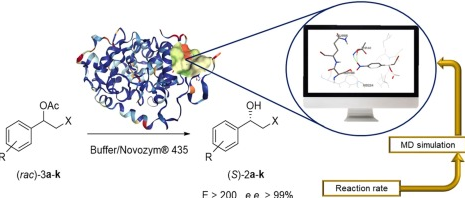
Lipase mediated enzymatic kinetic resolution of phenylethyl halohydrins acetates: A case of study and rationalization
Fonseca, T.D.S.| Vega, K.B.| da Silva, M.R.| de Oliveira, M.D.C.F.| de Lemos, T.L.G.| Contente, M.L.| Molinari, F.| Cespugli, M.| Fortuna, S.| Gardossi, L.| de Mattos, M.C.
Molecular Catalysis, 485, 110819 (2020)
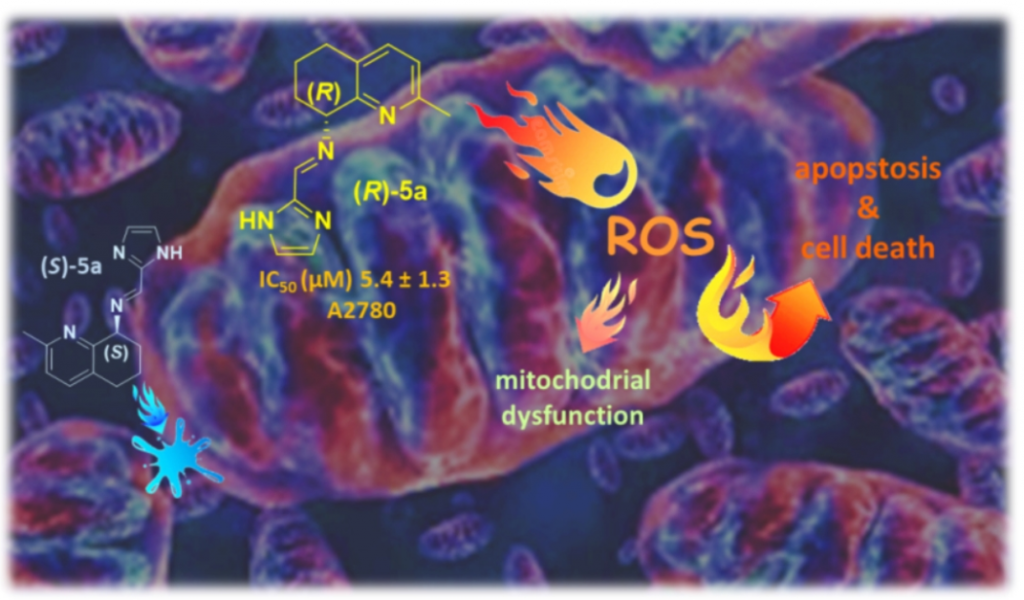
Biological properties of new chiral 2-Methyl-5,6,7,8-tetrahydroquinolin-8-amine-based compounds
Facchetti, G.| Christodoulou, M.S.| Mendoza, L.B.| Cusinato, F.| Dalla Via, L.| Rimoldi, I.
Molecules, 25(23), 5561 (2020)

Advances on whole-cell biocatalysis in flow
Pinto, A.| Contente, M.L.| Tamborini, L.
Current Opinion in Green and Sustainable Chemistry 25:100343 (2020)
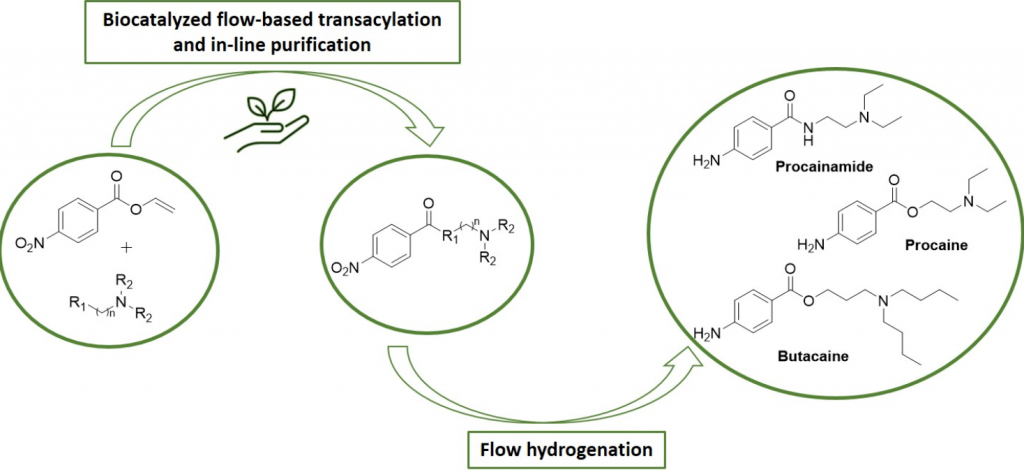
Efficient chemo-enzymatic flow synthesis of high value amides and esters
Annunziata, F.| Contente, M.L.| Betti, D.| Pinna, C.| Molinari, F.| Tamborini, L.| Pinto, A.
Catalysts, 10(8), 939 (2020)
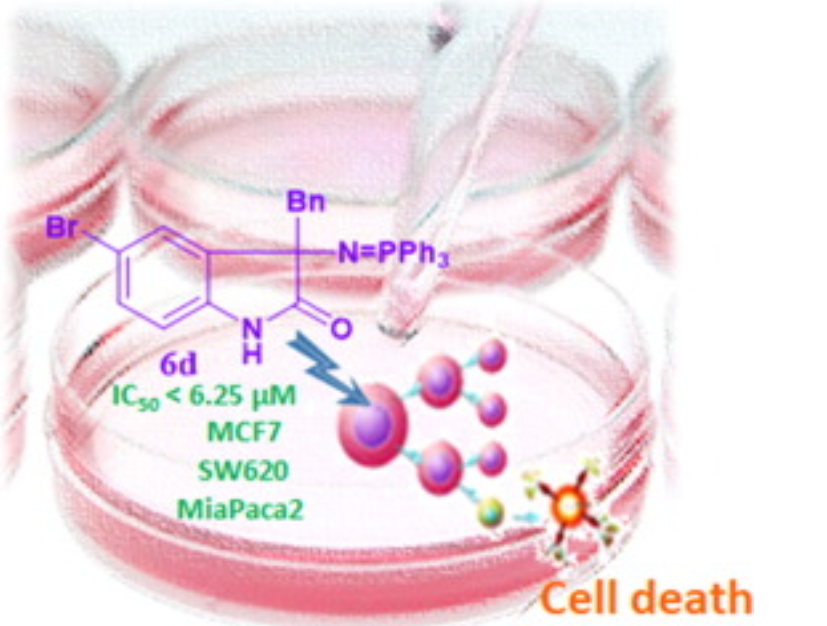
Novel 3,3-disubstituted oxindole derivatives. Synthesis and evaluation of the anti-proliferative activity
Christodoulou, M.S.| Nicoletti, F.| Mangano, K.| Chiacchio, M.A.| Facchetti, G.| Rimoldi, I.| Beccalli, E.M.| Giofrè, S.
Bioorganic and Medicinal Chemistry Letters, 30, 2, 126845 (2020)
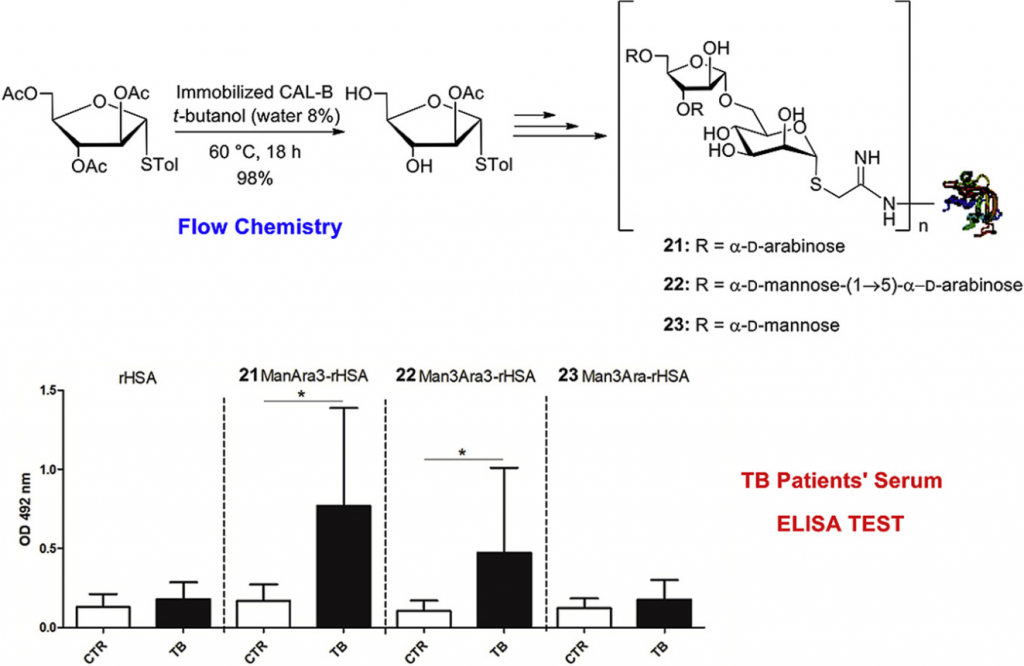
Chemoenzymatic synthesis of arabinomannan (AM) glycoconjugates as potential vaccines for tuberculosis
Li, Z.| Bavaro, T.| Tengattini, S.| Bernardini, R.| Mattei, M.| Annunziata, F.| Cole, R.B.| Zheng, C.| Sollogoub, M.| Tamborini, L.| Terreni, M.| Zhang, Y.
European Journal of Medicinal Chemistry 204, 112578 (2020)
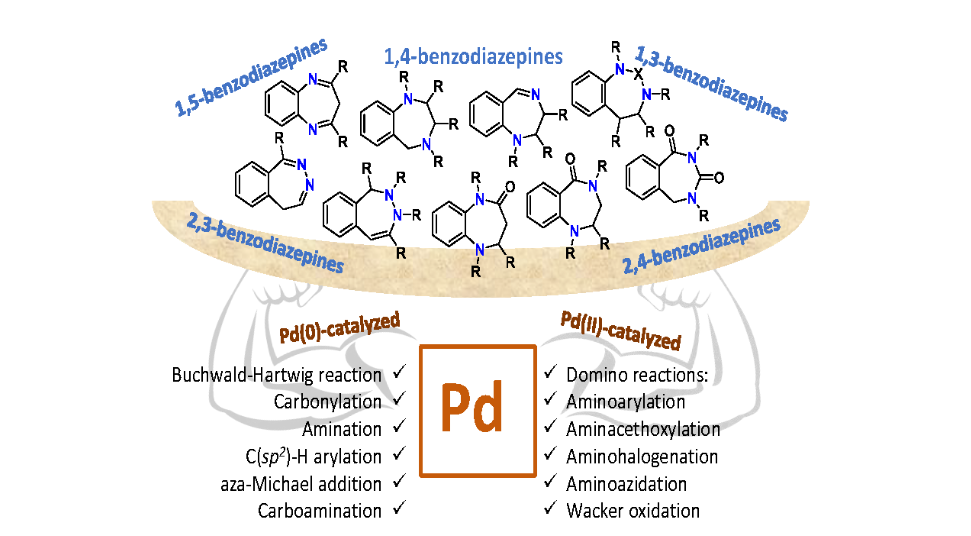
Palladium-catalyzed benzodiazepines synthesis
Christodoulou, M.S.| Beccalli, E.M.| Giofrè, S.
Catalysts, 10(6), 634 (2020)
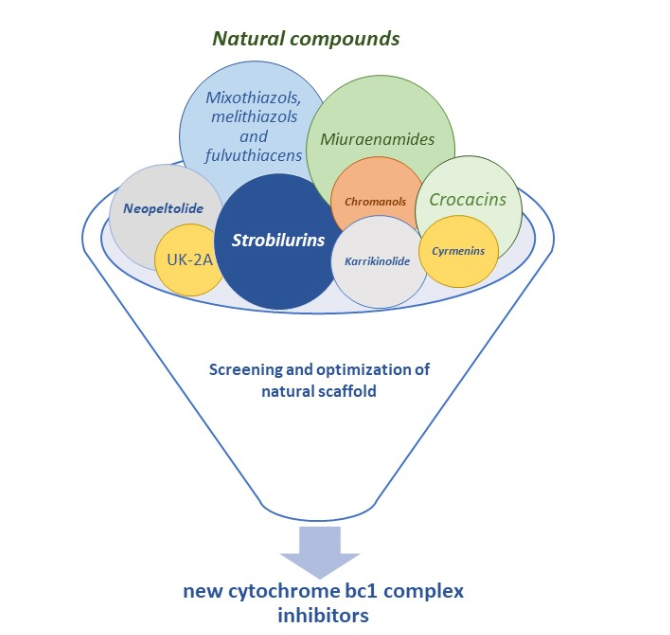
Natural compound-derived cytochrome bc1 complex inhibitors as antifungal agents
Musso, L.| Fabbrini, A.| Dallavalle, S.
Molecules 25(19), 4582 (2020)
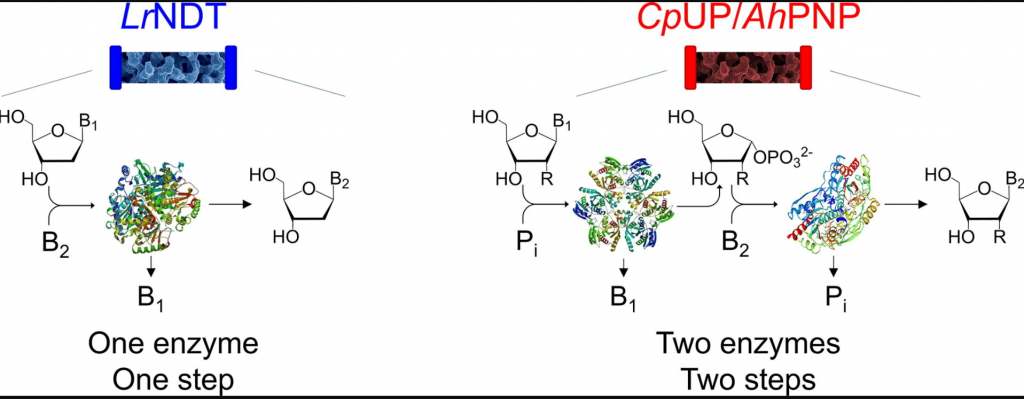
Immobilized enzyme reactors based on nucleoside phosphorylases and 2′-deoxyribosyltransferase for the in-flow synthesis of pharmaceutically relevant nucleoside analogues
Rinaldi, F.| Fernández-Lucas, J.| de la Fuente, D.| Zheng, C.| Bavaro, T.| Peters, B.| Massolini, G.| Annunziata, F.| Conti, P.| de la Mata, I.| Terreni, M.| Calleri, E.
Bioresource Technology 307, 123258 (2020)
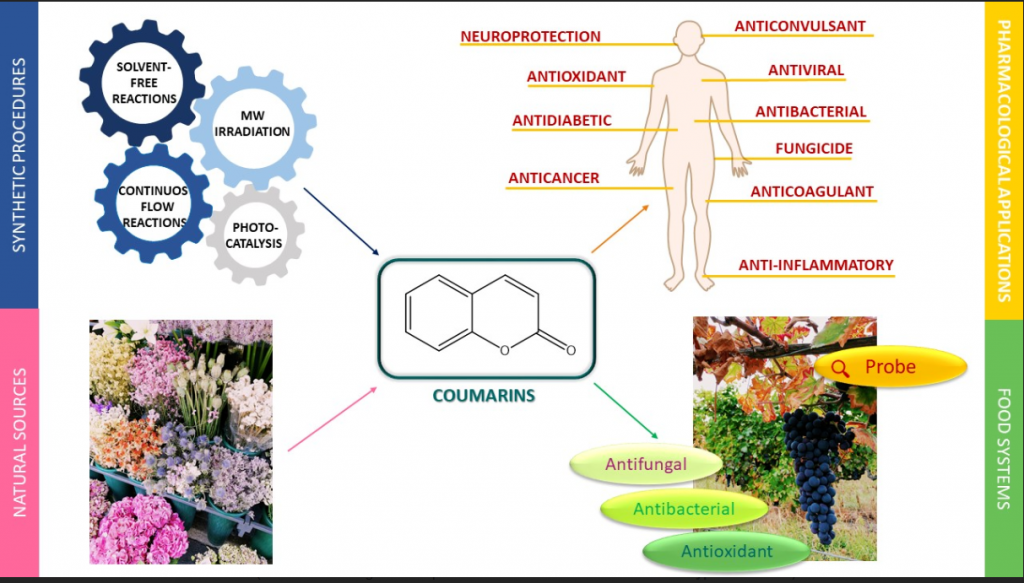
An overview of coumarin as a versatile and readily accessible scaffold with broad-ranging biological activities
Annunziata, F.| Pinna, C.| Dallavalle, S.| Tamborini, L.| Pinto, A.
Int. J. Mol. Sci., 21(13), 4618 (2020)

Antiproliferative effects of chalcones on T cell acute lymphoblastic leukemia-derived cells: Role of PKCβ
Corsini, E.| Facchetti, G.| Esposito, S.| Maddalon, A.| Rimoldi, I.| Christodoulou, M.S.
Arch Pharm., 353:e2000062 (2020)
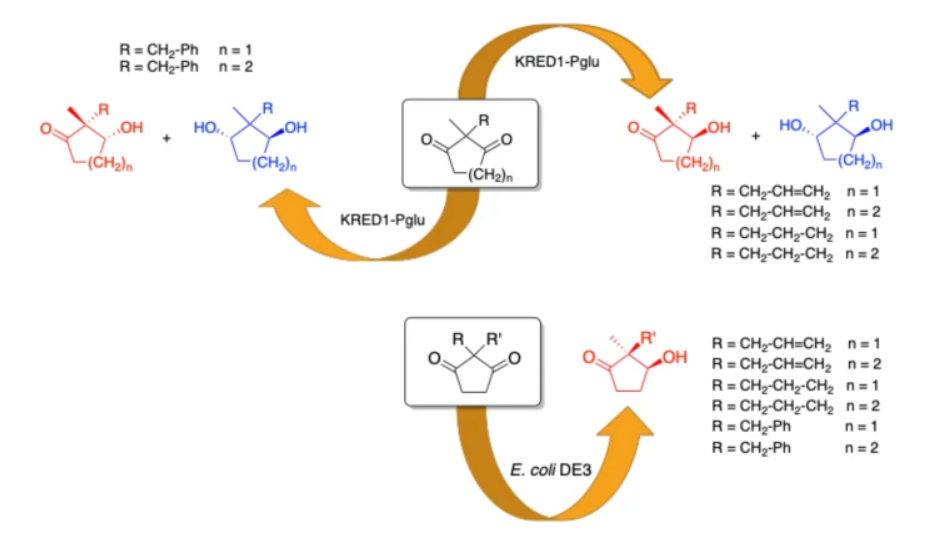
Stereoselective Reduction of Prochiral Cyclic 1,3-Diketones Using Different Biocatalysts
Contente, M.L.| Dall’Oglio, F.| Annunziata, F.| Molinari, F.| Rabuffetti, M.| Romano, D.| Tamborini, L.| Rother, D.| Pinto, A
Catal Lett 150, 1176–1185 (2020)
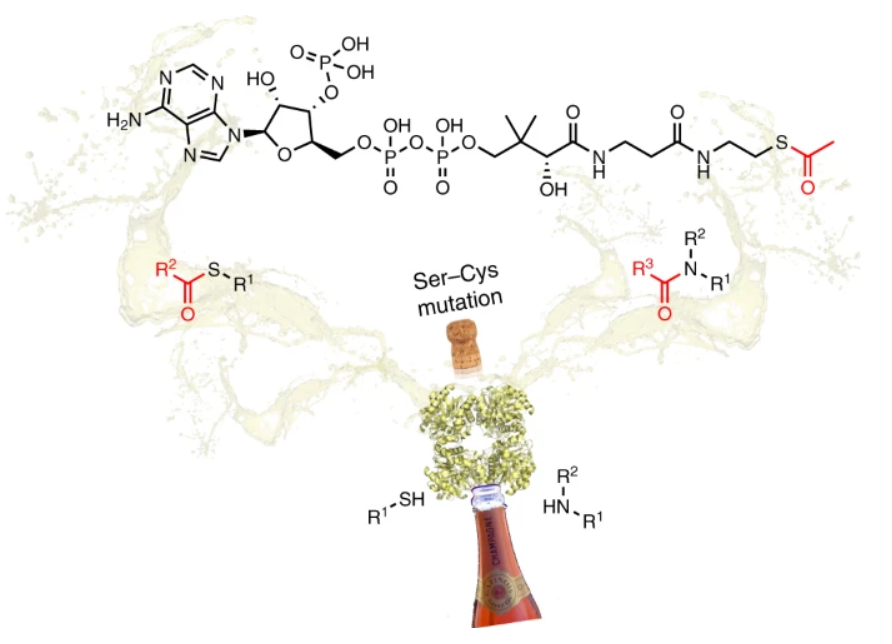
A strategic Ser/Cys exchange in the catalytic triad unlocks an acyltransferase-mediated synthesis of thioesters and tertiary amides
Contente, M.L.| Roura Padrosa, D.| Molinari, F.| Paradisi, F.
Nat Catal 3, 1020–1026 (2020).

Aromas flow: eco-friendly, continuous, and scalable preparation of flavour esters
Contente, M.L.| Tamborini, L.| Molinari, F.| Paradisi, F.
J Flow Chem 10, 235–240 (2020).
